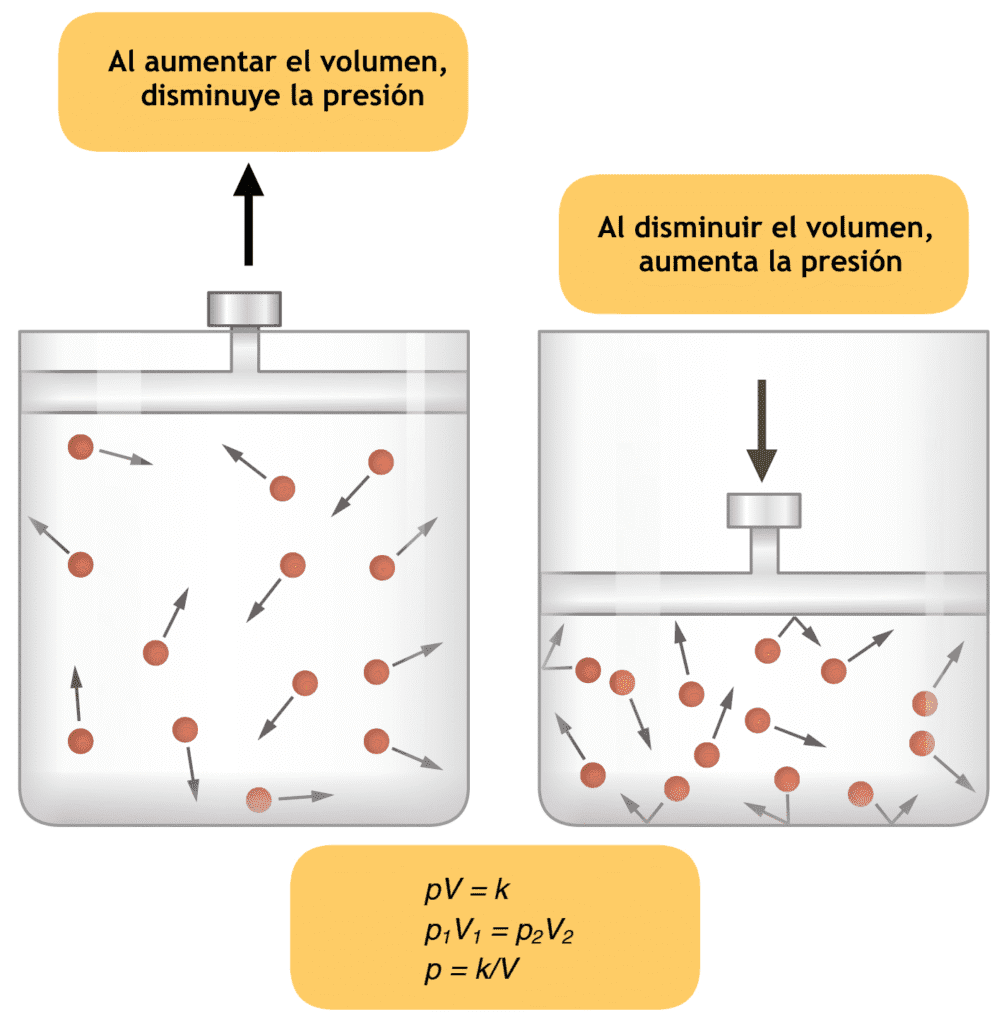
Charle’s law
Charle’s Law: A Fundamental Gas Law What is Charle’s Law? Charle’s Law is a fundamental principle in thermodynamics that describes how gases behave under varying conditions of temperature and volume. Formulated by Jacques Charles in the late 18th century, this law states that the volume of a gas is directly proportional to its absolute temperature, […]