Boyle’s law is an important gas law that states that at a constant temperature, the volume of gas is inversely proportional to the pressure of the gas. This means that when the temperature of a gas is kept constant, the relationship between Volume and pressure of a gas is inverse.
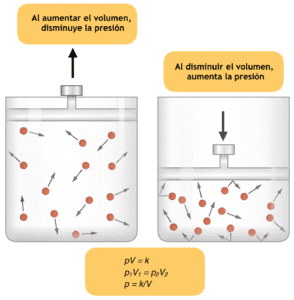
Another way to put tis law is to say that with any increase in the pressure of a gas at a constant temperature, the volume of the gas decreases. Similarly with any decrease in the pressure, while maintaining a constant temperature, the volume of the gas will increase.
What is Boyle’s Law?
Boyle’s Law is a fundamental principle in gas physics that describes the relationship between the pressure and volume of a gas at constant temperature. According to this law, when the volume of a confined gas decreases, its pressure increases, and vice versa. This relationship is crucial for understanding various phenomena in physical sciences and engineering.
The Formula of Boyle’s Law
The Boyle’s Law formula is expressed mathematically as PV = k, where P represents the pressure of the gas, V signifies its volume, and k is a constant that varies depending on the amount of gas and the temperature conditions. This equation illustrates how pressure and volume are inversely proportional; as one increases, the other must decrease to maintain the balance defined by the constant k.
Applications of Boyle’s Law
Boyle’s Law has numerous applications across various fields. In engineering, it’s essential for the design of pressurized systems, such as in scuba diving equipment or syringes. In meteorology, the law helps in understanding how changes in altitude affect atmospheric pressure. Furthermore, it plays a significant role in predicting how gases behave under varying conditions, enabling scientists and engineers to make informed decisions based on the gas’s properties.
Further more any increase or decrease in the volume of the gas at a particular temperature will decrease and increase the pressure of the gas respectively.
The law forms the basis of the relationship between the three important parameters of a gas i.e. Volume, Pressure and Temperature.
The knowledge of this law is also important in anesthetic practice, to understand the functioning of the Boyle’s machine which is the basic anesthesia machine and also the functioning of the oxygen and nitrous cylinders.
The two other laws apart from Boyle’s law that are essential to understand gas physics are the Charle’s law and the Graham’s law.

